1. Ionic bond | Definition, Properties, Examples, & Facts - Britannica
Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. The atom that loses the electrons becomes ...
Ionic bond, type of linkage formed from the electrostatic attraction between oppositely charged ions in a chemical compound. Such a bond forms when the valence (outermost) electrons of one atom are transferred permanently to another atom. Learn more about ionic bonds in this article.

2. Ionic and Covalent Bonds - Chemistry LibreTexts
Jan 22, 2023 · Ionic bonding is the complete transfer of valence electron(s) between atoms. It is a type of chemical bond that generates two oppositely charged ...
There are many types of chemical bonds and forces that bind molecules together. The two most basic types of bonds are characterized as either ionic or covalent. In ionic bonding, atoms transfer …

3. Lesson 4.5: Energy Levels, Electrons, and Ionic Bonding
Sep 8, 2023 · But in ionic bonding, electrons are transferred from one atom to the other and not shared like in covalent bonding.
American Chemical Society: Chemistry for Life.

4. 3.6 Electronegativity and Bond Polarity - Chemistry LibreTexts
Missing: terms | Show results with:terms
See AlsoA Factory Is Emitting Large Amounts Of Cfcs Into The Atmosphere. How Might This Affect People?When Magnesium Is Burned In Air, Large Amounts Of Heat And Light Are Given Out. Is This An Example Of An Exothermic Or An Endothermic Reaction?La structure la plus responsable du maintien de l’homéostasie cellulaire est laBond polarity and ionic character increase with an increasing difference in electronegativity. The electronegativity (χ) of an element is the relative ability of an atom to attract electrons to …

5. Chemical bonds | Chemistry of life | Biology (article) - Khan Academy
When one atom loses an electron and another atom gains that electron, the process is called electron transfer. Sodium and chlorine atoms provide a good example ...
Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Khan Academy is a nonprofit with the mission of providing a free, world-class education for anyone, anywhere.

6. The Chemical Components of a Cell - Molecular Biology of the Cell - NCBI
This electron exchange can be achieved either by transferring electrons from one atom to another or by sharing electrons between two atoms. These two strategies ...
Matter is made of combinations of elements—substances such as hydrogen or carbon that cannot be broken down or converted into other substances by chemical means. The smallest particle of an element that still retains its distinctive chemical properties is an atom. However, the characteristics of substances other than pure elements—including the materials from which living cells are made—depend on the way their atoms are linked together in groups to form molecules. In order to understand how living organisms are built from inanimate matter, therefore, it is crucial to know how all of the chemical bonds that hold atoms together in molecules are formed.
7. Which terms describe two atoms when they form a bond in ... - Numerade
Mar 24, 2023 · ... they form a bond in which electrons are completely transferred ... bond in which electrons are completely transferred from one atom to the other?
VIDEO ANSWER: Hello students in this question a statement is given. We have to give the correct term for that statement, which is suitable. The statement is 2 …
8. Ionic Compounds - University of Hawaii at Manoa
Ionic bonding occurs through a process called electron transfer, where one atom gives electrons to another. Imagine two puppies. One of the puppies has a bone ( ...
Electrons are in constant motion outside of an atom’s nucleus. The electron shell is the region that the electrons travel in (see Fig. 2.21). Electron shells are labeled with numbers 1 through 7. Each shell holds an increasing number of electrons, beginning with electron shell 1, which holds a maximum of two electrons (see Table 2.6).
9. Lewis Structures and the Shapes of Molecules - Angelo State University
Atoms tend to form covalent bonds in such a way as to satisfy the octet rule, with every atom surrounded by eight electrons. (Hydrogen is an exception, since it ...
Web page for Kevin A. Boudreaux, Chemistry Instructor at Angelo State University
10. [PDF] Chemical Bonding and Molecular Geometry
In ionic compounds, electrons are transferred between atoms of different elements to form ions. ... one central atom, the molecular structure completely describes ...
11. Ionic Bond (Electrovalent Bond) - Definition, Properties ... - BYJU'S
A chemical bond is formed between two atoms by the complete transfer of one or more electrons from one atom to the other as a result of which the atoms ...
Ionic Bond (Electrovalent Bond) - Ionic bonding involves the electrostatic interaction between oppositely charged species. Ionic bonds arise as cationic and anionic components of protein such as cationic and anionic species are found as acidic and basic groups.
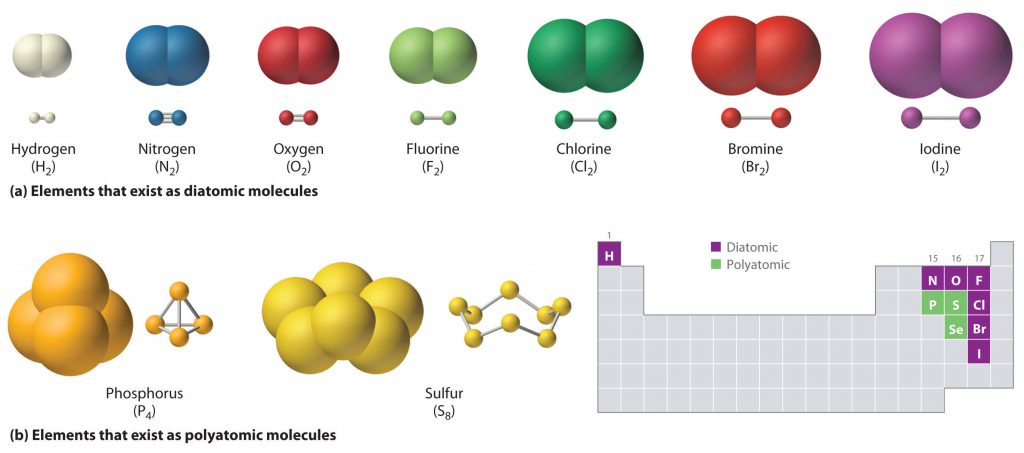
12. CH150: Chapter 4 - Covalent Bonds and Molecular Compounds
For example, one molecule of water would contain two hydrogen atoms and one oxygen atom (H2O). Chemists frequently use Lewis electron dot diagrams to represent ...
Chapter 4 – Covalent Bonds and Molecular Compounds This text is published under creative commons licensing, for referencing and adaptation, please click here. 4.1 Introduction to Covalent Molecules and Compounds How to Recognize Covalent Bonds 4.2 Electron Sharing Single Covalent Bonds Between the Same Atoms Single Covalent Bonds Between Different Atoms Multiple Covalent Bonds Coordinate […]
13. Valence Electrons - The Covalent Bond
The term covalent bond is used to describe the bonds in compounds that result from the sharing of one or more pairs of electrons. Return to Top of Page. How ...
The Covalent Bond
14. [PDF] Lesson 2 Bonding Bonding The atoms of many elements can combine to ...
single bond, so if two atoms share one, two or three electron pairs, then they are said. Page 2. to be joined by a single, double or triple covalent bond. A ...